Nagoya City Science Museum
TOP > Exhibition Guide > Keyword Search > Starting with "G" > graphite > Carbon
Carbon
Purpose of Exhibition
There exist 100 million different substances on earth, Many of which are substances containing carbon. They are composed of a combination of elements such as carbon, hydrogen, oxygen, and nitrogen.
How can a variety of substances be produced with so few elements? Watch the exhibit and imagine various molecules and chemical bonding.

Additional Knowledge
[Substances composed of only carbon]
Substances composed of only one element _ carbon - are diamonds, graphite (used for graphite pencil leads, etc.), fullerene (C60), carbon-nano-tube, amorphous -carbon (coal and soot), and so on. Those similar carbon elements have different connection methods. This is typical of carbon elements, and with various other elements.
By the way, the carbon nano-tube was discovered in 1991 by Sumio Iijima, and as a new material, attracted a lot of attention.
[Carbon has four ways of bonding]
Atoms are made up of electrons around a nucleus.
The number of electrons of each atom is equal to the atomic number.
The number of electrons that can enter each orbit trajectory is fixed.
Close to the nucleus, the number of electrons that can enter is 2, 8, 18, 32, 50, 72.
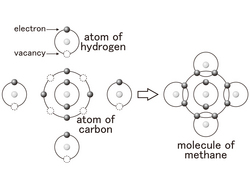
Now, the carbon atomic number 6 contains 2 electrons in inner orbit, and four electrons in the outer orbit.
When the number of electrons in the outer orbit is 8, it become stable, therefore there is room for four more. Carbon atoms fill the vacancy of four and share electrons and transform by creating other "molecules". This kind of bond is called "covalent bond".
The diagram shows a bond of one carbon and four hydrogen molecules.
Since the carbon shares four electrons, it's like four hands shaking each other.
Because of this, carbons that are alike lead off in various directions, producing a myriad of different molecules.
[What substance is C4H10O?] One example is the molecular formula C4H10>OThat is how many substances can be produced with four carbon, ten hydrogen, and one oxygen molecule. Please consider what the rule might be for bonding of four carbon, one hydrogen, and two oxygen molecules.
Various combinations are possible: please see the exhibit.
As in the example, different molecules made up from the same molecular - that is, the same atoms - are called "isomers"Because of the isomers, the compounds of carbon type do not increase further.
[Enantiomers]
Within isomers, there are substances which have a symmetrical molecular structure like mirrors. These are called "enantiomers".
There are two kinds of substances, like "left hand" and "right hand".
The only difference is the nature of light; boiling point and melting point are the same with the same density and solubility, similar nature and so on.
The two cannot be separated. We just wanted to artificially synthesize other substances only. They are surely created together.
However, we can distinguish organisms between the two:Only necessary ones can be made in the body.
For example, some "menthol" smells like mint, some doesn't.
In the exhibit, two types of menthol can be smelled. Please compare the fragrances.
These substances were first discovered in France in 1848 by Pasteur.
Since then, it is said that only living creatures have been able to separate it.
This common belief was broken by Ryoji Noyori's research.
He figured out how to create a method to artificially divide the smell of menthol completely, and won the 2001 Nobel Prize in Chemistry.
Article by Keiko Ishida, curator
