Nagoya City Science Museum
TOP > Exhibition Guide > Keyword Search > Starting with "P" > propane > Various Molecules and Chemical bond
Various Molecules and Chemical bond

Purpose of Exhibition
Molecule models of substances in our daily life are exhibited here. Some molecules consist of a few atoms and some are comprised of some hundreds of thousands of atoms. Pay attention to the correlation between commonality and propensity in each molecule's size, shape, and group of molecules.
The crystal models of metallic bond, ionic bond, and covalent bond are also on display.

Additional Knowledge
[Molecules]
Everything around us, including ourselves, consists of atoms. These atoms do not exist as an individual atom. Together with other atoms, they form a group as a molecule.
A water molecule is comprised of two hydrogen atoms and one oxygen atom. But if a water molecule is decomposed into hydrogen atoms and oxygen atom, it no longer has the propensity of water. Due to the structure of a molecule with some atoms, the propensity of the substance is determined.
Although there are about 112 kinds of atoms, depending on how they bond with each other, myriad kinds of molecules can be formed. The molecules introduced in this exhibition are only a handful of the total number.
We will introduce "Hydrocarbon in Methane Series" and give an explanation from the exhibit.
[Hydrocarbon in Methane Series]
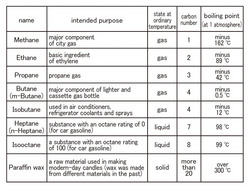
Hydrocarbon in methane series is often harnessed as a fuel that can be found in petroleum and natural gas. It is a compound shown as CnH2n+2 (n=1, 2, 3…). Among them, the eight kinds of molecules are exhibited here. (Chart1)
[Methane and Propane]
Are you aware that the bigger the number of carbon C, the higher the boiling point? (The temperature at which liquid becomes gas.)For the big molecules with big numbers of carbon, the stress on and between molecules grows larger. Therefore, it is difficult for molecules to remove the stress and pop into the air. In other words, they turn into gas. As a result, the boiling point increases.
Additionally, when a molecule becomes gas, the bigger the molecule is, the larger the density is; in short, the molecule gets heavy. The methane gas in town gas (Natural gas) is lighter than air, but there is a possibility that propane gas is heavier than air so it amasses near the bottom of the floor. We need to have good ventilation.
[Butane and Isobutane]
There is an ordinary cassette gas bottle with butane and one primarily with isobutane for outdoor activities in winter in cassette gas bottles. The boiling point mentioned above is the temperature for 1atm and both butane gases are liquefied in the high-pressured bottles at room temperature. By the way, temperature outside in winter hits nearly -0.6degree in Celsius which is butane's boiling point. Butane remains liquefied and not much gas is emitted. Therefore, fire becomes weakened. On the other hand, since the boiling point of isobutene is -12degree in Celsius, it means that good fire can be enjoyed.
Although each of the butanes is 4 in the number of carbons, they have very different boiling points. When the number of carbon is the same, a molecule with branching structure (isobutane) has a lower boiling point. Compare the shapes with the molecule models in the exhibit.
[Heptane and Isooctane]
Many substances are mixed in petroleum. A lot of heptane and isooctane is contained in petroleum and is the standard of an octane rating.
Octane rating is the octane number of a fuel showing how unlikely abnormal combustion occurs in a car engine (anti-knock), which is called knocking. Given that heptane, whose combustion rate is way too high and knocking is most likely to occur, is numbered 0, the isooctane most unlikely to cause knocking is numbered 100. Compare the mixtures of heptane and isooctane combined with many different ratios and petroleum you would like to examine. The ratio (volume %) of isooctane in the mixtures showing the same anti-knock is defined as octane ratingCompare these two molecules in the exhibit. Although the number of digits of the carbon is different, the boiling points are almost the same. The only difference is that heptane has a straight line-like structure and isooctane has a tree branch-like structure.
The structural difference has a lot to do with the propensity of a substance.
Article by Keiko Ishida, curator
