Nagoya City Science Museum
TOP > Exhibition Guide > Keyword Search > Starting with "F" > firefly > Fluorescence
Fluorescence


Purpose of Exhibition
You can see exhibitions that show shining colors (fluorescence) by ultraviolet light. Though ultraviolet is invisible light, the fluorescent material absorbs ultraviolet, and the visible light (visible radiation) is emitted. We see the color of it.
Fluorescent material is used in our daily lives such as fluorescent lights and plasma televisions. You will find them at unexpected places in this exhibition compared to where they are used in our daily lives.
Additional Knowledge
[The Phenomenon of the material that glows from within] There are two types of phenomena that demonstrate this glow from within.
(1) The light of the thermal radiation, which is from high-temperature solids and liquids. The light is emitted depending on the temperature and has no relation to the kind of the material. When the temperature is low, the color is simply infrared rays, and as the temperature gets higher, the light color changes to orange, yellow, white and blue-white. An incandescent lamp and firelight are good examples.
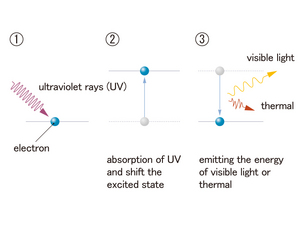
(2) Particular colored light is emitted depending on the materials. The atoms and electrons in the molecule get energy and enter an unstable high energetic state (excited state). When they return to the former energetic state (ground state), the surplus energy is emitted as light.
We will explain in detail about (2) by classifying the energy according to what kinds of energy it absorbs, with specific examples.
A. Electric energy: neon sign, mercury lamp, LEDB. Thermal energy: flame reactionC. Chemical energy: Light of firefly, sea firefly and yellow white phosphorus, luminol reaction and chemical light stickD. Light energy: fluorescence
[The Mechanism of the Fluorescence]
"Fluorescence" is D of (2) in the explanation above. Fluorescence is not like the light of the firefly. It is the phenomenon where light is reradiated as light energy from the materials that absorb X-ray, ultraviolet and visible radiation.
The fluorescence material of the exhibition emits a small amount of radiant energy by absorbing the ultraviolet, that is, light which has a lot of energy. There is a variety of radiant energy. The descending order of color is violet, cyan, blue, green, yellow, orange and red. The color from each fluorescence material is decided by the corresponding energy.
Like this, fluorescence has a characteristic in which the reemission of light energy is smaller than the absorption. It is because fluorescence emits light from molecules. Different from the discrete atom, the oscillatory motion and the rotational motion occurs between atoms in molecules, and the part of light energy absorption turns into thermal energy.
Some exhibited objects continue to emit light after the emission of ultraviolet is stopped. This phenomenon is called "phosphorescence". The phosphorescent material gradually emits the light and slowly goes back to the ground state.
[The Use of Fluorescence Material 1 - Fluorescent Whitening Agent - ]When a white cotton shirt gets old, it turns yellow. It looks yellow because the blue light in the incandescent light is absorbed. The fluorescent whitening agent is blended for the powdered detergent to make up for the blue light. The fluorescent whitening agent absorbs ultraviolet sunlight and the yellow tint is removed by the blue light (fluorescence). Therefore, the shirt looks pure white.
[The Use of Fluorescence Material 2 - Fluorescence Light - ]
There are some mercury gases in the tube of fluorescence light and fluorescence material is coated inside of the tube. When voltage is applied, the electron comes out from the electrode, bumps into the mercury atom and emits ultraviolet light. This ultraviolet light is absorbed into the fluorescence material and releases visible radiation.
Article by Keiko Ishida, curator
