Nagoya City Science Museum
TOP > Exhibition Guide > Keyword Search > Starting with "T" > Table of the Elements > Periodic Table of the Elements
Periodic Table of the Elements

Purpose of Exhibition
Currently, 118 kinds of elements are recognized internationally. They are ordered by their atomic number in the "periodic table of the elements" and are arranged in vertical lines. In this exhibition, an actual case of a periodic element, a single element and actual example of use is presented, which basically demonstrates what the periodic element table is composed of.The "periodic table search" allows you to examine the details of each element from the periodic table and element name with the touch panel.Also, you can examine what kind of element there is in the body, (explore from), And look for typical elements (search group).

Additional Knowledge
[Chemical Symbol]
In the early 19th century, an English chemist, John Dalton announced the elements' chemical symbols.
Then, a Swedish man, Berzelius, proposed the use of Latin as a symbol for the initials of each element. For example, O from oxygenium, and H from Hydrogen, and in the case of Hydrogenium, which has the same 'H', it is written with another letter, Hg.
[Atoms and Elements]
Atoms are "the basic particles that compose the material", a real particle.
Elements are the classification of atoms (number of protons). Element is a concept that represents the type of atom.
For example, hydrogen atoms exist in various states, such as hydrogen gas, water, and ethanol.
The number of protons of hydrogen atoms is one. The number of neutrons is zero or one or two. All those states are included in the concept of the hydrogen element.
A simple substance is made up of only one element. For example, hydrogen gas is a simple substance of hydrogen. Water is made of hydrogen and oxygen. In this case, the simple substance called hydrogen gas is not contained in the substance called water. When saying "Hydrogen" you should think if it's an element name or a simple substance name. For example, when saying milk contains calcium, it means that it contains some elements with calcium compounds.
The simple substance calcium is a silvery metal; this metal is not included in milk.
Elements, rather than something concrete like atoms, are abstract concepts.
[Atomic structure and periodic table]
All elements with atomic numbers are arranged (in order of approximate atomic weight) in the table. It is said to be a guide map of the elements, created to arrange all elements in line according to their similarities. By the way, when putting elements in order of atomic numbers, elements with similar properties appear at regular intervals (i.e. periodically). The reason lies in the structure of the atoms.
Atoms are made of protons and neutrons and electrons turning around it.
The atomic number attached to the element is equal to the number of protons in the atoms, and the number of protons is equal to the number of electrons.
Now, electrons are divided into several layers, which are called electron shells.
From the inner electron shell, 2, 8, 18, 32..., a fixed number of electrons can enter, the maximum capacity is fixed. The electron shell is packed from the inner section in order from low energy.
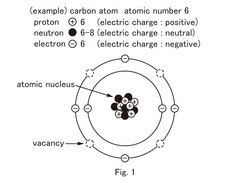
In the case of the carbon atom, with 6 electrons, two are on the inside and four electrons in the outside electron shell. (See Figure 1)The element written just below carbon in the periodic table is silicon.
The 14 electrons of silicon are contained in order within the electron shell 2, 8, 4. Then, the outermost electrons shell electrons (called the valence electrons) are the same four carbon elements.
In this way, the valence electrons of the elements arranged vertically on the periodic table are equal.
Chemical reactions occur because of interaction between atom valence electrons.
Elements with the same number of valence electrons have the similar chemical properties.
Article by Keiko Ishida, curator
