Nagoya City Science Museum
TOP > Exhibition Guide > Keyword Search > Starting with "M" > material > Metals
Metals

Purpose of Exhibition
Materials of industrial products are classified into three categories such as "metal", "ceramic", and "organic material". In this exhibition, the screen image with comments and real samples are displayed so that you understand "metal" and "plating".

Additional Knowledge
[Metallic bond and Metallic Property]
"Metal" is a crystal agglomerate whose metal atoms are orderly aligned. Common characteristics of metal are that it transmits electricity and heat smoothly, it has malleability (expand when pulled), ductibility (lengthen when metal is pulled), and metallic luster. These properties are the "metallic bond", when a metal atom shares free electrons as a whole. Conduction of electricity and heat are easy because free electron moves freely in the metal.
When the metal is hit and transformed, the atomic bond is maintained by free electron even if the position of the atomic bond is changed. That's why it can be changed and processed without breaking. Ductility of gold is especially large and it can be enlarged and transformed into gold leaf with the thickness of one ten thousandth. The metal surface of free electrons reflects all visible spectrums and it keeps its silver metallic luster. In the case of gold or copper, it absorbs parts of the visible spectrum and the color becomes red or yellow.
[Iron]
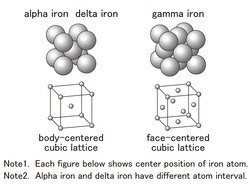
About 70 types of elements are metallic elements in nature. Within metals, iron is 30 percent of the weight of the earth. Iron ore, which is a material of iron, exists as iron oxide. Iron is made by causing a chemical reaction between iron oxide and coke under high temperature and getting rid of the oxygen. Here, pure oxygen is too soft and not useful. Typically, steel is an alloy containing less than 2 % carbon or other elements. Steel has a carbon component of under 2 percent, and it is called cast iron when containing 2 to 6.7% carbon. [Steel and Heat Treatment] The alignment of iron atoms (crystal structure) changes according to the temperature. In the case of pure iron, it has three structures as illustrated in the drawing: alpha iron at a 910 degrees or lower temperature, gamma iron at a temperature between 910 and 1400 degrees, and delta iron at a temperature between 1400 and 1535 degrees. When steel is heated to 800 to 900 degrees and chilled as slowly as possible, it becomes soft steel. This process is called annealing. However, when steel is heated then chilled rapidly in water or oil, hard, brittle steel is produced. This process is called quenching. Why does the rate of cooling cause such differences in their properties? While steel is hot and has the structure of gamma iron, carbon atoms are positioned in a lattice of iron atoms. When such steel is chilled slowly, it will try to change its structure to alpha iron. In the alpha iron structure, however, iron atoms are arranged more tightly and carbon atoms cannot stay in a lattice of iron atoms. For this reason, carbon atoms move slowly to produce two types of finely segmented parts, alpha iron consisting solely of iron atoms and iron-carbon compounds Fe3C.
This is the structure of steel formed after an annealing process, which is characterized by softness and ductility.
On the other hand, in the quenching process, in which steel is chilled rapidly, carbon atoms have no time to move. So, an irregular structure with carbon atoms forced into the arrangement of iron atoms in alpha iron is produced instead. When steel with such a structure is subject to an external force working to deform it, it does not yield to such force, because intervening carbon atoms prevent it from deforming. In other words, steel has become hard. To become hard, however, means that steel is vulnerable to shocks and breaks easily. To overcome this fault, quenched steel is heated to 400 to 600 degrees again for tempering. Tempered steel shows hardness, yet better toughness (does not break easily).
Cooperation: Nippon Steel CorporationDaido Steel Co., Ltd.
Japan Aluminium AssociationAichi Plating Industrial Association, Illustrations and Article by Ishida Keiko, Curator
