Nagoya City Science Museum
TOP > Exhibition Guide > Keyword Search > Starting with "S" > surface tension > Super-Hydrphobic and Super-Hydrophilic
Super-Hydrphobic and Super-Hydrophilic

Purpose of Exhibition
When dropping water droplets on a frying pan that is processed with fluorine-containing resin, the droplets become round and rise due to surface tension. This property that repels water is referred to as hydrophobicity. On the other hand, when dropping water droplets on a clean glass plate, the droplets neither get round nor raise so much, and spread flat instead. Thus, the property of getting wet is called hydrophilicity.
In this exhibition, you can actually drop water droplets on superhydrophobic and superhydrophilic materials and observe what happens. You can also see enlarged footage (that was taken beforehand) of the experiment.
Additionally, both of the technologies that are introduced in this exhibition are learnt, researched and developed by a biological mechanism.
Additional Knowledge
[Water Repellant Surface and Water Absorbing Surface]
The difference between a water repellant surface and water absorbing surface is whether or not water and a material will get along. Fluorine-containing resin cannot go with water and they are against each other. On the other hand, glass and water have a good relationship. That is why glass gets wet.
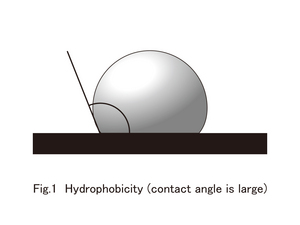
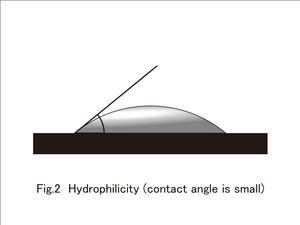
By the way, the degree of water repellency and water absorbency depends on the surface of a material and the contact angle of a water droplet. Although there is no clear definition, generally, the case of a contact angle more than 90 degrees is termed as superhydrophobicity (water repellant or difficult to get wet)(figure 1), and the when the contact angle is less than 40 degrees it is called Superhydrophilic(water absorbing) (figure 2).
[Superhydrophobicity]
Generally, when the surface of a material and the contact angle of a water droplet are over 150 degrees, it is called superhydrophobic.
You have probably seen dewdrops that roll over and over on the leaves of a lotus or aroid leaf after it rains. While the contact angle between a fluoroplatsic and a water droplet is about 120 degrees, the angle of a lotus leaf is about 160 degrees and it is superhydrophobic. Why do leaves of a lotus repel water that much?Though the surface of a lotus leaf seems to be flat, it has some_ microscopic roughness on it. Moreover, on the surface of each roughness, there are some hundredths of 1 _ microscopic juts. This double-roughness structure holds circular dewdrops.
The superhydrophobic processing introduced in the exhibition is developed by researching the surface structure of a lotus leaf. Made with organic silicon compound as a raw material, the microscopic-roughness structure is created by piling up the very small particles like snow.
The conventional waterproofing spray is not superhydrophobic, but is simply put on a material (absorbed). Therefore, it has no durability. Whereas this superhydrophobic processing makes durability improve, since chemically bonded with the material, it is also useable for window glass because it is transparent. Though the application to a car windshield is under study, cars without wipers might become a possibilty in the future.
[Superhydrophilicity]
The case in which the contact angle between a material surface and a water droplet is very small is called superhydrophilic. The silica component is applied on the surface of a material that is on display in this exhibition here. A silica component goes well with water and is superhydrophilic. It absorbs moisture in the air strongly and makes a thin film of water. Even if dirt (oil dirt) sticks on it, it is flushed together when water is poured. Since this method does not require light, it plays a good role even in a poorly lit place. This technology is in use for external walls of buildings and houses where cleaning is difficult. When it rains, dirt comes off easily and the walls can be kept clean.
This mechanism of preventing dirt was developed by the study of the mystery as to why a snail shell is always clean. When observing a snail shell with an electron microscope, tiny ditches are spread on the surface of the shell. It always keeps water in them. In other words, the whole shell is covered by a thin film of water at all times. Dirt is flushed with rain water, and that is the reason why a snail shell is always clean.
Living things are amazing. It seems like there are many more things that we can learn from nature.
(By the way, other than methods introduced in this exhibition, there is also a method using the photocatalysis reaction in superhydrophobic processing).
Cooperation:
TAKEDA PRINTING CO.,LTD.
Takai Osamu, Professor, Department of Materials, Physics and Energy Engineering, Graduate School of Engineering, Nagoya University.
INAX Corporation(now LIXIL Corporation)
Article by Keiko Ishida, curator
